
The Nucleus is Composed of Protons and Neutrons The radius of an atom must be defined arbitrarily, such as the boundary in which the electron can be found with 95% probability. The latter has no definite outer boundary, so neither does the atom. It is common (but somewhat misleading) to describe the volume of space in which the electrons of an atom have a significant probability of being found as theĮlectron cloud. Its behavior in terms of the probability of its manifesting itself at any point in space. The extremely small mass of the electron (1/1840 the mass of the hydrogen nucleus) causes it to behave as a quantum particle, which means that its location at any moment cannot be specified the best we can do is describe
#DEFINE ATOMIC NUMBER HOW TO#
This section describes how to determine the average atomic mass of an element if we know the isotope masses and their relative abundance. Then read the section "Isotopic Mixtures and Abundances" near the bottom of the page. When we see the mass of an element on the periodic table, we are seeing the weighted average of the masses of all isotopes of that element. Read this section, which explains how we write atomic symbols with atomic numbers and mass numbers. We can write this as A = Z + N where A is mass number, Z is atomic number, and N is number of neutrons.
#DEFINE ATOMIC NUMBER PLUS#
For a given isotope, we define the mass number, A, as the atomic number plus the number of protons. However, the number of neutrons can vary within atoms of a given element.Ītoms of the same element with different numbers of neutrons are called isotopes. For a neutral atom (not a charged ion), the number of electrons must equal the number of protons.

We define the atomic number, Z, as the number of protons in an atom.
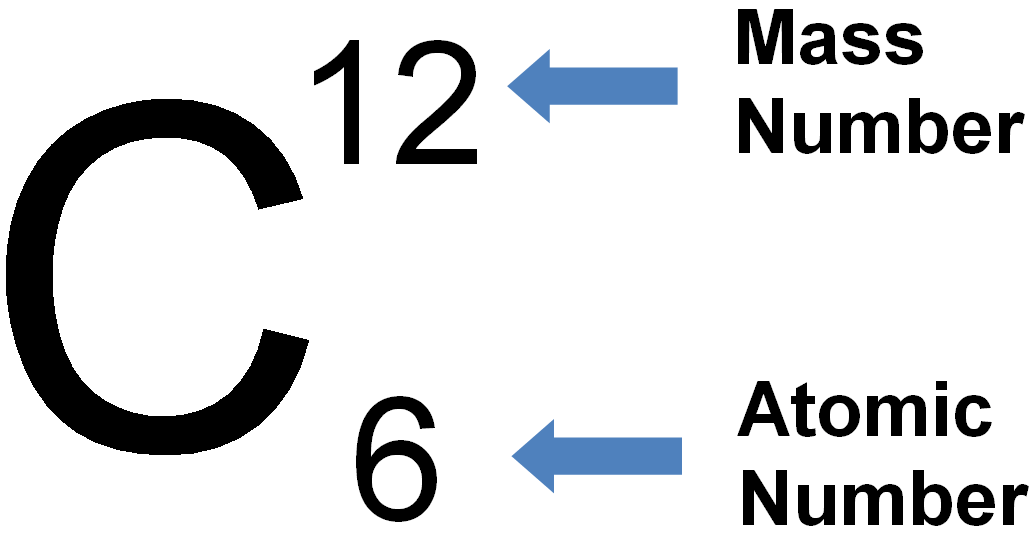
As stated above, we define the elements by their number of protons.


 0 kommentar(er)
0 kommentar(er)
